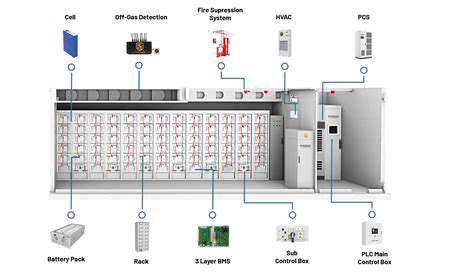
Beyond Lithium Ion: Emerging Energy Storage Solutions

Flow Batteries: A Powerful Energy Storage Solution
Flow batteries represent a promising advancement in energy storage technology, offering a compelling alternative to traditional battery chemistries. Their unique design allows for significantly higher energy storage capacities compared to lithium-ion batteries, making them particularly suitable for large-scale applications. This scalability is crucial for meeting the growing demands of renewable energy integration. Their modular nature facilitates the expansion of storage capacity as needed, adapting to fluctuating energy production from sources like solar and wind.
Mechanism of Operation: A Detailed Overview
Unlike traditional batteries, flow batteries store energy chemically in separate electrolyte tanks. These electrolytes are pumped into electrochemical cells, where electrochemical reactions occur, storing or releasing energy. This unique separation of the electrolyte and electrochemical process provides several advantages, including enhanced safety and longer lifespan compared to traditional batteries. The separation also allows for the possibility of using different electrolyte combinations to optimize the battery's performance and characteristics.
Advantages over Conventional Batteries
Flow batteries boast a number of advantages over conventional battery technologies. They exhibit a substantially longer lifespan, often exceeding 10,000 cycles, which is a significant improvement compared to the limited cycle life of lithium-ion batteries. Their inherent safety profile is another key advantage, as the separation of electrolytes reduces the risk of thermal runaway and other safety concerns. This translates to a lower risk of catastrophic failures, offering a more reliable storage solution.
Applications and Future Potential
The versatility of flow batteries makes them suitable for a wide range of applications. Their large-scale energy storage capabilities make them ideal for grid stabilization and supporting the integration of intermittent renewable energy sources. These batteries are also well-suited for stationary energy storage applications, such as in industrial facilities and large-scale data centers. Further research and development are expected to unlock even more potential applications, potentially including electric vehicle storage, where their high energy density and long lifespan could prove advantageous.
Environmental Impact and Sustainability
The environmental impact of flow batteries is a significant consideration. Many flow battery chemistries utilize environmentally benign materials, reducing the environmental footprint compared to some other battery technologies. Their ability to use existing infrastructure for electrolyte storage and transport also contributes to a more sustainable solution. The long lifespan of these batteries further reduces the need for frequent replacements, contributing to a lower overall environmental impact throughout their entire lifecycle.
Metal-Air Batteries: Harvesting the Power of the Atmosphere
Harnessing Atmospheric Oxygen
Metal-air batteries represent a compelling alternative to lithium-ion batteries, promising higher energy density and a more sustainable future for energy storage. Their unique characteristic lies in their ability to utilize atmospheric oxygen as the cathode, a readily available and environmentally benign resource. This inherent feature dramatically reduces reliance on scarce and often environmentally problematic materials, a significant step forward in the quest for sustainable energy solutions. The process of oxygen reduction reaction (ORR) at the cathode is critical in these systems, and ongoing research focuses on optimizing this process for enhanced efficiency and longevity.
The abundance of oxygen in the atmosphere presents a significant advantage. Instead of relying on finite resources like lithium, metal-air batteries offer a potentially limitless supply of oxidant. This has profound implications for the future of portable electronics, electric vehicles, and stationary energy storage systems, potentially revolutionizing how we power our world.
Electrochemical Fundamentals
The fundamental principle behind metal-air batteries hinges on electrochemical reactions. These reactions involve the transfer of electrons between the anode and the cathode, creating an electric current. The anode, typically made of a metal like zinc or aluminum, undergoes oxidation, releasing electrons. Simultaneously, oxygen from the air is reduced at the cathode, accepting these electrons. Understanding and controlling these processes is key to optimizing battery performance.
The intricate interplay of redox reactions at the electrode surfaces is a crucial aspect of metal-air battery technology. Further research into catalysts and electrode materials is vital to improve the efficiency and durability of these batteries, leading to practical applications that are both effective and sustainable.
Challenges and Opportunities
Despite their promise, metal-air batteries face several challenges, primarily concerning their cycle life and power density. Issues like corrosion, the formation of passivation layers, and the difficulty in achieving high rates of charge/discharge cycles can hinder their widespread adoption. Overcoming these hurdles is essential to unlocking the full potential of this technology.
Conversely, the opportunities are vast. Significant research efforts are focused on developing advanced materials, optimizing electrode designs, and improving electrolyte formulations. This is driving innovations in areas like sustainable energy storage, portable electronics, and electric vehicles, potentially leading to a paradigm shift in how we power our modern world.
Material Science Innovations
A critical aspect of metal-air battery development is the exploration of novel materials. Researchers are investigating different metal alloys, novel catalysts, and advanced electrolytes to enhance performance metrics. The search for materials that can effectively catalyze the oxygen reduction reaction is a major focus, as it directly impacts the efficiency and lifespan of the battery.
Applications and Future Directions
The potential applications of metal-air batteries extend beyond portable electronics. Their high energy density makes them attractive for electric vehicles, stationary energy storage, and even portable power sources for remote areas. Future research should focus on addressing the issues of battery lifetime, improving the cycle life of the battery, and reducing the cost of materials for wider implementation. The development of more robust and efficient metal-air batteries could revolutionize energy storage and pave the way for a greener energy future.
The potential for large-scale implementation is significant. Success in this area could directly impact the transition to a low-carbon economy and provide sustainable energy solutions for various applications, from personal electronics to large-scale grid storage.
Other Emerging Technologies: Exploring Novel Approaches
Beyond the Batteries: Exploring Solid-State Batteries
Solid-state batteries are poised to revolutionize energy storage, offering significant advantages over lithium-ion technology. These batteries utilize solid electrolytes, which enhance safety and potentially increase energy density. This innovative approach mitigates the flammability risks associated with liquid electrolytes, a significant concern in lithium-ion batteries, while also potentially boosting the overall performance. The development of high-performance, stable solid electrolytes remains a critical challenge, but ongoing research holds great promise for a future where solid-state batteries power our devices and vehicles more efficiently and safely.
Harnessing the Power of Supercapacitors
Supercapacitors, also known as ultracapacitors, offer a unique energy storage mechanism different from traditional batteries and capacitors. They provide rapid charge and discharge capabilities, making them ideal for applications requiring frequent power cycling. Their fast charging and discharging cycles make them exceptionally suitable for applications such as hybrid electric vehicles, where quick power delivery is crucial. Supercapacitors also exhibit a longer lifespan compared to conventional batteries, adding to their appeal for various applications.
Furthermore, their lightweight design and relatively low cost compared to some battery alternatives make them an attractive option for portable electronics and other applications.
The Rise of Flow Batteries: A Sustainable Solution
Flow batteries represent a promising approach to large-scale energy storage. These systems store energy chemically in separate liquid electrolytes, enabling the storage of substantial amounts of energy in a modular fashion. This modularity is particularly beneficial for grid-scale energy storage, allowing for flexible expansion and adaptation to changing energy demands. Flow batteries exhibit a longer lifespan compared to traditional batteries and offer a promising path towards sustainable energy solutions.
Exploring the Potential of Fuel Cells: Clean Energy for the Future
Fuel cells offer a pathway towards clean and efficient energy production. They directly convert chemical energy from a fuel source (such as hydrogen) into electricity, producing water as a byproduct. This process inherently reduces emissions, making fuel cells an attractive option for various applications, from portable electronics to large-scale power generation. The development of more cost-effective and efficient fuel cell technologies is crucial for widespread adoption.
The Impact of Graphene and Other Advanced Materials
Graphene, a revolutionary material with exceptional electrical and mechanical properties, is rapidly emerging as a critical component in various energy storage and conversion technologies. Its high surface area and excellent conductivity make it ideal for enhancing the performance of batteries, supercapacitors, and fuel cells. Other advanced materials, such as carbon nanotubes and metal-organic frameworks (MOFs), are also showing promise in enhancing energy storage capabilities in novel ways. The integration of these advanced materials promises to boost performance and efficiency across a broad range of energy-related applications.
Looking Ahead: Integrating Emerging Technologies
The future of energy storage and conversion lies in the integration of these emerging technologies. Hybrid systems combining different approaches, such as lithium-ion batteries with supercapacitors, can offer optimized performance tailored to specific applications. Research in areas such as materials science and advanced manufacturing will be essential to drive further innovation and cost reduction in these emerging technologies.
Addressing Challenges and Future Directions
Despite the exciting potential of these emerging technologies, several challenges remain. Cost-effectiveness, scalability, and safety considerations need to be addressed for wider adoption. Continued research and development efforts are crucial to overcome these hurdles and unlock the full potential of these novel approaches. The future direction of research will likely focus on developing more efficient and cost-effective materials, optimizing system designs, and addressing safety concerns associated with handling and storing these energy-dense technologies.