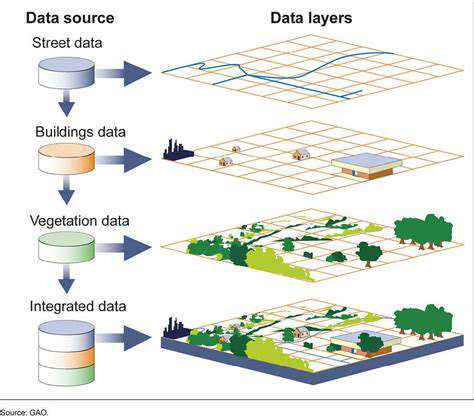
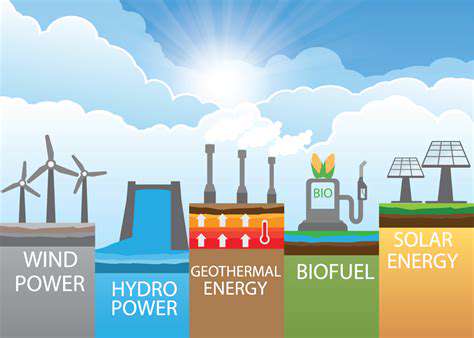
Advancements in Green Hydrogen Electrolysis Technologies
Solid Oxide Electrolysis (SOE): Emerging Potential for High-Temperature Applications
Understanding Solid Oxide Electrolysis (SOE)
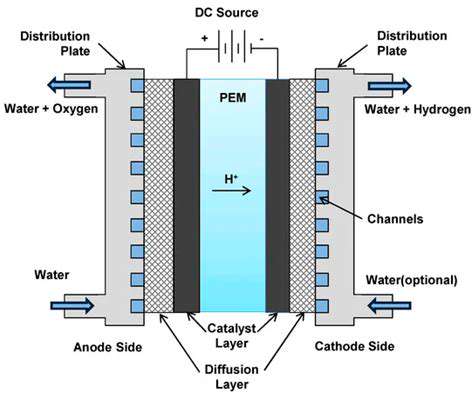
Solid Oxide Electrolysis (SOE) is an electrochemical process that utilizes high temperatures to decompose water into hydrogen and oxygen. This process, occurring within a solid oxide electrolyte, is a promising alternative to traditional methods for hydrogen production. The high-temperature environment allows for efficient water splitting, potentially leading to a lower energy consumption compared to some other electrolysis methods. It also offers the unique advantage of producing hydrogen with minimal carbon emissions, particularly if the electricity source is renewable.
SOE systems are composed of several key components, including the solid oxide electrolyte, electrodes, and a high-temperature environment. The electrolyte plays a crucial role in facilitating the ion transport of oxygen ions, while the electrodes catalyze the reactions at the interfaces. Understanding the intricate interplay of these components is vital to optimizing SOE technology.
High-Temperature Advantages
The high-temperature operation of SOE systems offers several advantages over lower-temperature alternatives. One significant benefit is the higher efficiency of water splitting at elevated temperatures, which translates into lower energy consumption for hydrogen production. Additionally, higher temperatures can enable the use of less expensive and more readily available materials in the SOE cell components, potentially reducing the overall cost of the technology.
Moreover, high temperatures can potentially improve the reaction kinetics, leading to faster hydrogen production rates. This is particularly important for industrial applications where high throughput is crucial.
Materials Considerations in SOE
The selection of materials for SOE components is critical for the long-term performance and stability of the system. Solid oxide electrolytes, typically composed of complex oxides, must exhibit high ionic conductivity at the operating temperature while maintaining structural integrity. Electrodes also require high catalytic activity for water splitting reactions and compatibility with the electrolyte and operating environment.
The choice of materials can significantly influence the efficiency, cost, and durability of the entire SOE system. Research and development efforts are focused on identifying and synthesizing new materials with improved performance characteristics.
SOE Applications in Industry
SOE technology has the potential to revolutionize various industrial sectors. Its ability to produce green hydrogen makes it attractive for applications such as fuel cell vehicles, ammonia production, and industrial processes requiring hydrogen as a feedstock. By coupling SOE with renewable energy sources, a sustainable hydrogen production pathway can be established.
The flexibility of SOE to integrate with diverse energy sources and its potential to support various industrial processes makes it a promising technology for a future powered by clean energy.
Challenges in SOE Development
Despite its promising potential, SOE technology faces several challenges. High operating temperatures require sophisticated materials and manufacturing processes, potentially increasing the cost of the system. Long-term stability and durability of the components at elevated temperatures remain key concerns. Furthermore, scaling up SOE systems from laboratory prototypes to industrial-scale implementations presents significant engineering and logistical obstacles.
Economic Viability and Market Potential
The economic feasibility of SOE depends on factors such as material costs, energy costs, and technological advancements. As the cost of renewable energy sources decreases and materials science progresses, SOE could become a more competitive option for hydrogen production. The market potential of SOE is significant, particularly in sectors with high hydrogen demand.
Government support and incentives for research and development, along with the increasing need for clean energy solutions, could accelerate the adoption and commercialization of SOE technology.
Environmental Impact and Sustainability
The environmental impact of SOE is crucial to its long-term viability. By using renewable energy sources to power SOE electrolysis, the production of hydrogen can be significantly decarbonized. This is a key factor in mitigating climate change and achieving a sustainable energy future. Furthermore, the potential for SOE to replace fossil fuel-based hydrogen production methods contributes to reducing greenhouse gas emissions.
The environmental benefits associated with SOE, coupled with its potential to support diverse industrial applications, make it a promising technology for a sustainable future.